■clay retorts suffer damage), as on the contrary, in the electric furnace, the lead
is distilled together with the zinc. In spite of the fact that the unit of
heat produced electrically is dearer than that produced from fuel, the electric
production of zinc has nevertheless been the object "of numerous experiments.
Among the few which have led to any result are those conducted by the famous
■Swedish inventor, O. de Laval, whose method has, however, now been modified.
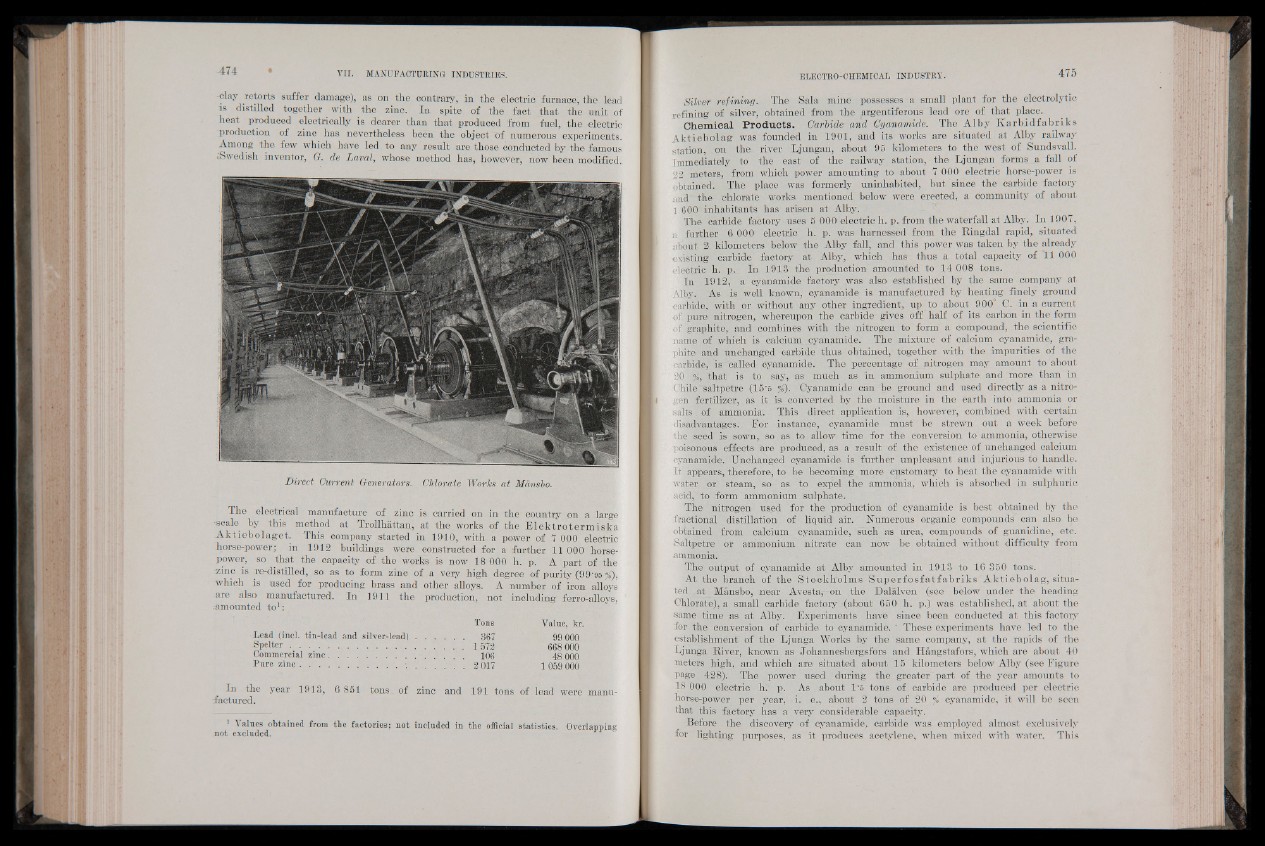
Direct Current Generators. Chlorate Works at Mdnsbo.
The electrical manufacture of zinc is carried on in the country on a large
■scale by this method at Trollhattan, at the works of the E lek tro te rm is'k a
A k t ie b o la g e t . This company started in 1910, with a power of 7 000 electric
horse-power; in 1912 buildings were constructed for a further 11 000 horsepower,
so that the capacity of the works is now 18 000 h. p. A part .of the
zinc is re-distilled, so as to form zinc of a very high degree of purity (9 9 ‘96 %),
which is used for producing brass and other alloys. A number of iron alloys
■are also manufactured. In 1911 the production, not including ferro-alloys,
■amounted to1:
Tons Value, kr.
Lead (incl. tin-lead and silver-lead)............... 367 99 000
■Spelter...................................................................... . 1 5 7 2 668000
Commercial zinc 106 48 000
Pnre z in c ........................2017 1 059 000
In the year 1913, 6 851 tons. of zinc and 191 tons of lead were manufactured.
1 Values obtained from the factories; not included in the official statistics. Overlapping
not excluded.
Silver refiningjj The Sala mine possesses a small plant for the electrolytic
refining of silver, obtained from the argentiferous lead ore of that place.
Chemical P r o d u c ts. Carbide• and Gyanamide. The A lb y K a rb id fa b r ik s
A k tie b o la g was founded in 191)1, and its works are situated at Alby railway
station, on the river Ljungan, about 95 kilometers to the west of Sundsvall.
Immediately to the east of the railway station, the Ljungan forms a fall of
22 meters, from which power amounting to about 7 000 electric horse-power is
obtained. The place was formerly uninhabited, but since the carbide factory
and the chlorate works mentioned below were erected, a community of about
1 600 inhabitants has arisen at Alby.
The carbide factory uses 5 000 electric h. p. from the waterfall at Alby. In 1907,
u further 6 000 electric h. p. was harnessed from the Ringdal rapid, situated
about 2 kilometers below the Alby fall, and this power was taken by the already
existing carbide factory at Alby, which has thus a total capacity of 11 000
electric h. p. In 1913 the production amounted to 14 008 tons.
In 1912, a cyanamide factory was also established by the same company at
Alby. As is well known, cyanamide- is manufactured by heating finely ground
carbide, with or without any other ingredient, up to about 900 C. in a current
of pure nitrogen, whereupon the carbide gives off half of its carbon in the form
of graphite, and combines with the nitrogen to form a compound, the scientific
name of which is calcium cydnamide. The mixture of calcium cyanamide, graphite
and unchanged carbide thus obtained, together with the impurities of the
carbide, is called cyanamide. The percentage*' of nitrogen may amount to about
20 % that is to say, as much as in ammonium sulphate and more than in
Ghile 'saltpetre (15‘6 yfyt Cyanamide can be ground and used directly as a nitrogen
fertilizer,. as it is converted by the moisture in the earth into ammonia or
salts of ammonia. This direct application is, however, combined with certain
disadvantages.. Eor instance, cyanamide must be strewn out a week before
the seed is sown, so as to allow time for the conversion to ammonia, otherwise
poisonous effects are produced, as a result of the existence of unchanged calcium
cyanamide. Unchanged cyanamide is further unpleasant and injurious to handle.
It appears, therefore, to be becoming more customary to heat the cyanamide with
water or steam, so as to expel the ammonia, which is absorbed in sulphuric
acid,' to form ammonium sulphate.
The nitrogen used for the production of cyanamide; is' best obtained by the
fractional distillation of liquid air. Numerous organic compounds' can also be
obtained from calcium cyanamide, such as urea, compounds of guanidine, etc.
Saltpetre or ammonium nitrate can now be obtained without difficulty from
ammonia.
The output of cyanamide at Alby amounted in 1913 to 16 350 tons.
At the branch of the Sto.ckh'olms S u p e r f o s f a t f a b r ik s A k t ie b o la g , situated
at Mansbo, near A vesta, on the Dalalven (see below under the heading
Chlorate), a small carbide factory (about 650 h. p.) was established, at about the
same time as at Alby. Experiments have since been conducted at this factory
for the conversion of carbide to cyanamide. « These experiments have led to the
establishment of the Ljunga Works by the same company, at the rapids of the
Ljunga River, known as Johannesbergsfors and Hangstafors, which are about 40
meters high, and which are situated about 15 kilometers below Alby (see Figure
page 428). The power used during the greater part of the year amounts to
18 000 electric h. p. As about 1*6 tons of carbide are produced per electric
horse-power per year, i. e., about 2 tons of 20 % cyanamide, it will be seen
that this factory has a very considerable capacity.
Before the discovery of cyanamide, carbide was employed almost exclusively
for lighting purposes, as it produces acetylene, when mixed with water. This