il ‘fe.
I toi
I
O)
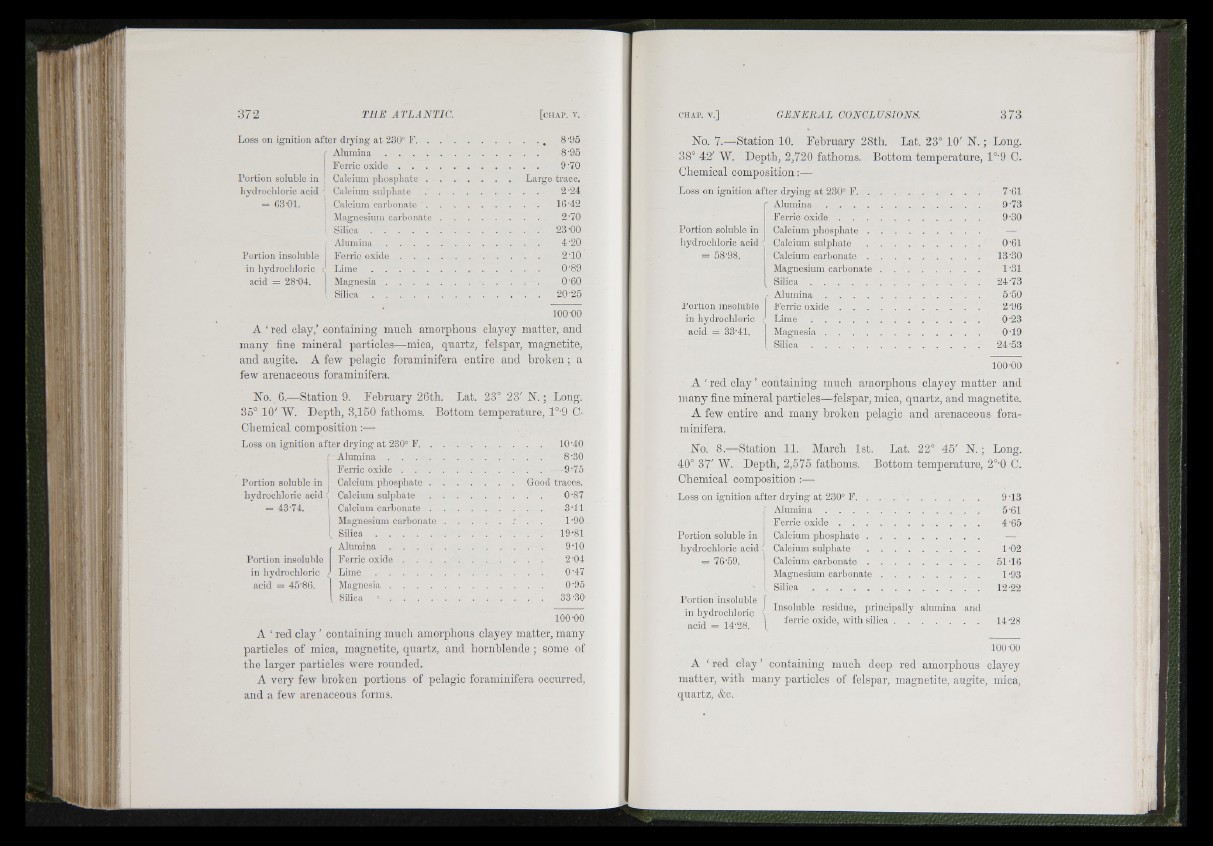
Loss on ignition after drying a t 230° F.
Portion soluble in
hydrochloric acid
= G3-01.
Portion insoluble
in hydrochloric
acid = 28'04.
Alumina . . . .
Ferric oxide .
Calcium phosphate .
Calcium sulphate
Calcium carbonate .
Magnesium carbonate
S i l i c a ........................
Alumina . . . .
Ferric oxide .
L i m e ........................
Magnesia . . . .
S i l i c a ........................
Largì
100-00
A ‘red clay,’ containing much amorphous clayey matter, and
many fine mineral particles—mica, quartz, felspar, magnetite,
and augite. A fe-w pelagic foraminifera entire and b ro k en ; a
few arenaceous foraminifera.
No. 6.—Station 9. February 26th. Lat. 23° 23' N. ; Long.
35° 10' W. Depth, 3,150 fathoms. Bottom temperatnre, 1°'9 C-
Chemical composition:—
Loss on ignition after drying a t 230° F .................................................... 10-40
Alumina ............................................... 8-30
Ferric o x i d e ........................................................... 9-75
Calcium p h o s p h a te .........................................Portion soluble in Good traces.
hydrochloric acid
= 43-74.
Portion insoluble
in hydrochloric
acid = 45-86.
Calcium sulphate
Calcium carbonate .
Magnesium carbonate
S i l i c a ........................
Alumina . . . .
Ferric oxide .
. L i m e ........................
Magnesia . . . .
.............................. 0-87
.............................. 3-11
. . . . . . 1-90
.....................................19-81
.............................. 9-10
............................. 2-04
............................. 0-47
............................. 0-95
Silica - ................................................................ 33-.30
100-00
A ‘ red c lay ’ containing mnch amorphous clayey matter, many
particles of mica, magnetite, quartz, and hornblende ; some of
the larger particles were rounded.
A very few broken portions of pelagic foraminifera occurred,
and a few arenaceous forms.
Depth, 2,575 fathoms.
Chemical composition :—•
Loss on ignition afte r drying a t 230° F,
Alumina
Portion soluble in
hydrochloric acid :
= 76-59.
Portion insoluble
in hydrochloric
acid = 14-28.
Ferric oxide . .
Calcium phosphate
Calcium sulphate
C a lc ium c a rb o n a te ............................................... 51-16
IMagnesium c a r b o n a t e ......................................... 1-93
S i l i c a ...................................................................... 12-22
Insoluble residue, principally alumina and
ferric oxide, with s il ic a ........................................... 14-28
100-00
A ‘ red c la y ’ containing much deep red amorphous clayey
matter, with many particles of felspar, magnetite, augite, mica,
quartz, &c.